Quantitation of Biosurfactant
Biosurfactants can be quantified by surface and interfacial tension. This is a generic quantitation and thus does not distinguish among different types of surfactants that may be present. Biosurfactants can be compared in terms of the amount they reduce surface or interfacial tension, and the critical micelle concentration (cmc), which is the lowest surfactant concentration above which no further decrease in surface tension or interfacial tension takes place.
Measurement of Surface Tension
Surface tension measures the force required to move a ring immersed in a surfactant solution upward through the surface of the liquid into air. Surface tension in a sample is measured with a surface tensiometer, for example a Model 21 Tensiomat (Fisher, Pittsburgh, PA), which uses the du Nouy ring method.
Some medium components dissolved in solution, e g , glucose, do not interfere with the du Nouy ring method. Other medium components, such as peptone, or solvents, such as methanol, can reduce surface tension m the absence of a surfactant. Therefore, care must be taken to use the proper surface tension controls Also, some floating materials, such as froth or oil, can affect measurement of surface tension.
In this case, a separation may be required to remove the floating materials. Alternatively, biosurfactants may be quantified by methods other than surface tension. For example, rhamnolipids may be determined by measurement of L-rhamnose by the orcinol method.
Surface and interfacial tension measurements are dependent on temperature. Therefore, all samples should be equilibrated at room temperature before measurement.
The cmc is determined by measuring the surface tension in a series of samples diluted in 0.1 M, pH 7.0 phosphate buffer. A standard plot is made of log (surfactant concentration) vs surface tension and is used to estimate the cmc. Look at this graphic.
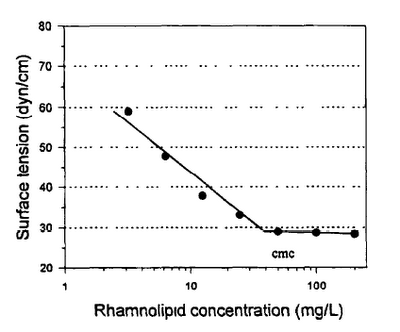 Fig. 1. Standard plot of surface tension against the log concentration of mono rhamnolipid. The surface tension was measured in 0.1 M, pH 7 0, phosphate buffer
Fig. 1. Standard plot of surface tension against the log concentration of mono rhamnolipid. The surface tension was measured in 0.1 M, pH 7 0, phosphate buffer
To determine the surfactant concentration in an unknown sample, dilute the sample until the surface tension measured is above the minimum surface tension (the surfactant concentration in solution is below the cmc). The concentration is then determined using a standard plot as shown in figure above and multiplying by the appropriate dilution factor.
In some cases, the identity of a biosurfactant is unknown and a simple screening of a crude surfactant solution is desired. The concentration of biosurfactant can, therefore, not be measured. Such crude surfactant solutions may be compared on the basis of surface tension.
Measurement of Interfacial Tension
Interfacial tension measures the force required to move a ring immersed in one liquid, in this case a hydrocarbon, upward through a liquid : liquid interface into a second liquid, in this case water. The procedure of determining surfactant concentration in a sample by measurement of interfacial tension is similar to measurement of surface tension.
Hopefully by reading this post you don’t confuse anymore in calculating the tension of biosurfactant and also its concentration.